Your circ-water piping: Is it a disaster waiting to happen?
By Thomas F Armistead, Consulting Editor
A circulating-water pipe failed at Unit 3 of Southwestern Public Service Co’s Harrington Station, Amarillo, Tex, late last October. No one was injured because the failure occurred in the evening, and the maintenance crew was gone, but the breached pipe flooded the basement with 3 in. of water and damaged the electrical circuit breakers. It forced all three units of the 1066-MW coal-fired powerplant to shut down because they share the basement.
The company declined to release the damage estimate, but it will be “a few million” dollars, said David Low, general manager of generation in Texas and New Mexico for Xcel Energy, SPS’s parent company. All three units were returned to service at the end of December.
Out of sight and out of mind, circulating-water pipe is easy to overlook in planning for maintenance during a scheduled outage. “The circulating-water system is the simplest critical system in the powerplant,” said John M Brodar, PE, mechanical/corrosion engineer at Salt River Project, Tempe, Ariz. But he emphasizes “critical.”
To Brodar, “critical” describes any component of a powerplant system that can interfere with the plant’s only function, which is producing megawatts. If a large, reputable, investor-owned utility can be blindsided by a failure of its circ-water system, every powerplant owner in the US should heed the warning.
Most critical powerplant systems—the fuel system, gas turbine, HRSG, and others—are much more complex than the circ-water system, which consists of piping, the condenser, and the cooling tower, with few moving parts to break down. These form a cooling loop, drawing water out of the cooling tower to the condenser, then returning it to the top of the cooling tower. Pressure is low, typically less than 50 psig. “Because there are so few problems, it’s easy to ignore it, forget about it,” Brodar said.
Threat and counterthreat
If the system is simple, and its problems are few, what should you be worrying about? You’re probably already maintaining the above-ground parts, the condenser and the cooling tower, but when did you last inspect and maintain the piping? The principal threats to the circulating-water pipe are physical damage during transport and installation, overburden loading on buried pipe, and corrosion.
Most circ-water pipe is made of steel, concrete, or plastic: fiber-reinforced plastic (FRP) or high-density polyethylene (HDPE). Each material has its strengths and weaknesses, which determine the applications for which they are used.
Point loading from surface live loads is a threat to buried pipe that can too easily be overlooked once the pipe is out of sight underground, and the various types of pipe differ in their ability to handle pressure. Steel, FRP, and HDPE handle internal pressure well, but not external pressure, especially when the pipe is empty or unpressurized. Concrete, on the other hand, is strong for external loads but easily overpressurized.
It is important to keep these characteristics in mind when designing buried piping that passes under driveways in a powerplant, for example, because extremely heavy equipment, such as transformers and turbines, must be delivered via road to the plant. If you’re unaware or forget that the pipe is under the road, the ultra-heavy load can damage it. Good design will anticipate that. “Where roads cross buried pipe, you can cause big problems to empty, unpressurized pipe during an outage if you drive a load over the buried pipe,” said Brodar.
HDPE is probably the most trouble-free material of all, “almost indestructible,” said Brodar. It is easily fabricated using automated welding fixtures to complete nearly perfect butt welds, which actually are stronger than the rest of the pipe. In 2008, the Nuclear Regulatory Commission endorsed the material’s reliability when it approved HDPE for safety-related water pipe, a first for the industry. And plastic pipe generally resists corrosion because it is chemically inert, Brodar said.
But thermal expansion can harm plastic pipes because the material has a high coefficient of thermal expansion, meaning that a thermal change will result in a higher axial displacement for plastic than for metal pipe. Water temperature above 150F in plastic pipe also could pose a threat. Extremely low ambient temperatures may cause hard thermoplastic materials to become brittle. FRP expansion, however, is less than HDPE’s. The strength, rigidity, and surface hardness of plastic pipe are inferior to steel pipe’s properties.
Plastic pipe is vulnerable to collapse caused by a vacuum, which can result from siphoning. Large FRP circ-water lines require wall ribbing, vacuum relief, or specific operating procedures to counter this threat.
As noted, plastic pipe resists corrosion, but FRP is not entirely immune, according to the National Association of Corrosion Engineers International, because of the way NACE defines corrosion. FRP has a gel coat for chemical resistance and water resistance, but if the gel coat is damaged, the glass fibers become exposed, and the water will wick along the fibers for a substantial distance (6-12 in.), weakening the bond between the fibers and the epoxy, and thus weakening the pipe, Brodar said.
It’s not corrosion of metal, but meets NACE’s definition of corrosion as “the deterioration of a substance, (usually a metal) or its properties because of a reaction with its environment.” From the outside, the pipe looks undamaged, but the effect is to weaken the pipe. In addition, FRP is brittle and vulnerable to pinpoint pressure. So, for FRP pipe that is buried, “bedding is incredibly important,” said Brodar. The bedding material must be entirely free of rocks.
Prestressed concrete cylinder pipe (PCCP) also is brittle, but it compensates with massive strength. It is a good choice for burial under railroads and roads that will carry heavy loads. Bedding requires less attention than plastic pipe’s because concrete is not as vulnerable to pinpoint pressure, and vacuum-caused collapse is not a threat.
The alkaline nature of the cement mortar protects the steel prestressing wires from corrosion, making the steel passive by shifting its pH well above 11, said Brodar. A crack in the coating, however, puts this type of pipe at risk by exposing the bare steel, making it the anode and accelerating corrosion. If that happens, PCCP can fail catastrophically, he said (Figs 1-3).


CorrosionUncoated steel and HDPE pipes are not vulnerable to pinpoint pressure, but corrosion can attack steel pipe both inside and out, said Brodar. Fusion-bonded epoxy, coal-tar enamel, polyethylene, or vinyl tape wrap will protect steel pipe against exterior corrosion, but these coatings also are susceptible to pinpoint damage, so the bedding for coated steel pipe should be similar to that for FRP. Bedding for both should be rock-free clean sand or screened native soil, but above the springline, the backfill for both can be rock-free, graded native soil.
Uniformity in the embedment is necessary because “corrosion is due to differences,” Brodar explained. The presence of different materials around a pipe creates a galvanic cell (Sidebar 1). A cell cannot affect plastic pipe, but it will corrode unprotected steel. The source of the sand also matters with steel-pipe embedment. “If you use beach sand, you introduce chlorides, and that will create problems,” said Brodar. Slag and mine tailings provide bedding as good as sand for compaction, but bad chemistry, with resulting corrosion. Cathodic protection is required to prevent corrosion of buried steel pipe by galvanic action.
A cathode and an anode in an electrolyte with a metal path connecting the poles create a galvanic cell. For buried pipe, a difference in embedment materials can cause anodes and cathodes to form on the same piece of pipe. Cathodic protection doesn’t stop corrosion, it just transfers it to the anode, whose only function is to corrode (Sidebar 2).
The coating on the pipe isolates it from the electrolyte, but if pinholes exist in the coating, the pipe will become anodic and corrosion will occur at the pinhole. For exterior corrosion protection, coatings and cathodic protection are synergistic, said Brodar. They are more effective together than either is alone.
Uniformity of embedment material is no guarantee against galvanic corrosion, however. Pipe that is buried in soil or partially immersed in groundwater is susceptible to corrosion, and near the ocean, saltwater intrusion creates other corrosion problems. Landfills also produce corrosive liquids.
Powerplant designers must take these environmental conditions into consideration when they design buried piping, whether for circ-water systems or other purposes. If you are unsure whether your buried pipes were designed with this in mind, or you have other reasons for thinking they may be at risk, now would be a good time to inspect them and make certain of their condition.
Inside the pipe, there are other potential sources of corrosion. Plastic pipe is chemically inactive, so it’s immune. Concrete pipe’s corrosion depends upon the pH of the water: If it’s high enough, the pipe can be unaffected for decades; if not, “the concrete can be very rapidly attacked,” said Brodar. The attack can destroy the pipe. “If normal pH is less than 8, the situation should be further evaluated,” he said. “Any pH excursion into the acidic range will cause permanent attack on the concrete pipe.”
Coated steel pipe will handle a broader pH range than concrete, depending on the interior coating, said Brodar. “Most epoxies can handle long-term exposures to from neutral (7 pH) to highly alkaline conditions. Excursions to a pH of 5 or even 4 should not cause major concerns,” he added. But if the coating is damaged, the pipe is even more susceptible to corrosion than concrete (Fig 4). “If a significant pH excursion has occurred, say, from a sulfuric acid addition error, expect every coating defect to become heavily contaminated with sulfates,” he said (Fig 5).
“Chemical decontamination with low-pressure water cleaning at 3500 psig minimum using a zero-degree rotating tip should be performed at the next outage on every coating defect,” followed by touch-up of the affected areas (Fig 6). “Cathodic protection can and will protect the exterior from corrosion if the CP system itself is maintained. Interior coatings can be maintained on pipe larger than 54 in.,” he added, “but below 54 in., access with blasting equipment is very difficult.”



Seawater corrosion on steel is typically about 5 mils per year, said Brodar, but he has seen corrosion from microbiological deposits as high as 50-75 mils per year. This is a key change in the corrosion rate from decades past. He says it appears to be concurrent with the change in recent years from liquid chlorine to other biocides in circ-water systems. That change has occurred because liquid chlorine is difficult to handle.Microbiological deposits can be a significant threat to the interior of a circ-water line because they greatly increase the corrosion. As the microbes grow, they form a gelatinous mass that isolates the microbe colony from the general environment. Some of these microbes will feed on the sulfates in the circulating water. The waste product is sulfuric acid, which will rapidly attack the steel.
The change in the last 10 years has been “dramatic,” Brodar said. For the first time ever, for example, he has seen through-wall pits at SRP’s 2409-MW Navajo Generating Station in Page, Ariz. On a recent inspection he saw a pit deeper than 325 mils, and he found 25 to 30 through-wall pits. With the latter, he noted, it’s impossible to say whether the corrosion originated inside the pipe or outside because the corrosion walls are perpendicular to the pipe wall.
The source of water in a circulating-water system makes a difference, Brodar said. Seawater is a known corrosion agent that coatings counteract. Fresh water is not nearly as corrosive in a once-through cooling system, but for a cooling tower, as cycles are increased, corrosion problems grow with concentration.
As in real estate, location also matters. East of the Mississippi, water tends to be acidic, west of it, alkaline. The use of industrial and municipal effluent in cooling-system makeup water also may result in increased corrosion because the micro-organisms that create biofilm feed on the nitrogen, phosphate, and organic compounds in the effluent.
Macrobiological organisms, principally zebra mussels and quagga mussels, also threaten circ-water systems. Their larvae are microscopically small, less than 200 microns, and can’t be filtered out of cooling water before they attach to the circ-water pipes and form colonies that grow and block the pipes. Condenser tubes or condenser inlets are ideal locations for these colonies. Even before they are large enough to block the pipes, mussels interfere with cooling-water flow and allow sediment buildup.
Powerplants in Europe deal with the mussels by having two identical circ-water system intakes so they can shut down one for cleaning while using the spare for continuing operation, Brodar said. The mussels are not yet a problem in SRP’s system, but he wondered why operators don’t backwash with hot water to kill the organisms. Temperatures over 120F halt biofouling, he said.
Even intact, uncorroded pipe can be threatened by leaks from pipe joints. Bell-and-spigot joints are used for concrete and FRP piping, and the American Water Works Association allows some leakage, Brodar noted. But you need to pay attention to the allowable leakage, he added. Soils will have different drainage characteristics. Joint leakage that could be acceptable in well-draining soils may become problematic in clayey soils. EPC contractors like to use packaged designs, and may overlook factors that will be important to your pipe. “Make sure the EPC addresses the soil conditions at your facility for his canned design,” he said.
Inspection, maintenance
Like other powerplant systems, circulating-water pipes require regular inspection and maintenance, but this requirement is often overlooked. A visual inspection of the cleaned interior coated surface is the first requirement. Any flaw in the coating requires further investigation.
All of the traditional nondestructive examination techniques can be used for steel circ-water systems, including ultrasound, magnetic particle, and liquid-penetrant inspection of steel to detect cracks. Radiographic inspection is generally not considered because it requires access from both sides of the pipe wall, and buried pipes are accessible only from the interior. For fiber-reinforced plastic, inspection is mainly a matter of looking for blisters, Brodar said.
Until fairly recently, the only way to inspect prestressed concrete cylinder pipe was by visual inspection or sounding—tapping the surface with an inspection hammer. Good concrete sounds solid when struck, and the hammer rebounds readily. Disbonded or weak concrete, on the other hand, gives a dull sound, a “thud.” Brodar now recommends a baseline remote-field eddy current (RFEC) for inspection on all critical PCCP. If the baseline survey identifies damaged reinforcing wires, then periodic follow-up surveys are required.
The eddy current send-receive probe technique is a screening tool, said Tom Burnett, power group director with Intertek Aptech, Houston, the contractor that inspected Southwestern Public Service’s Harrington Station after its circ-water pipe failed. The technique uses variations in the strength of magnetic waves shot through metal to identify locations of possible corrosion for visual inspection.
Brodar likes RFEC because it locates corrosion in the prestressing wires in PCCP. Aptech used pulsed eddy current testing at Harrington because the failed pipe was carbon steel, and PEC “can test relatively large areas without significant preparation, and provide a good screening evaluation of wall loss in ferritic steels,” says an Aptech report.
The exterior of buried circulating-water lines cannot be inspected without excavation. Because of this it is essential to maintain the cathodic protection system for the exterior of the lines. A simple close-interval potential survey is the easiest way to confirm adequate cathodic protection.
The interior is another story. Pipe size and length are the factors here. Pipe diameter affects inspection and maintenance mainly because, “if you can’t stand up in the pipe, it’s a real challenge to perform inspections and especially coating maintenance,” said Brodar (Fig 7). On a long pipe, manholes must be spaced so that hoses and cables will allow the workers to reach the midpoint between them.

The principle that rules large-diameter underground pipe maintenance is, “Whatever goes into the manhole has to come back out,” said Brodar. If the sandblasting operation uses 40 tons of sand, that’s 40 tons that must be removed at the end. Instead of hauling out buckets of sand through a manhole, he has had “phenomenal success” using a vacuum truck to clean out the sand. Sandblasting also requires “dragging a big, fat hose with fittings and ears across thin-film paint,” causing the invert, or floor of the pipe, to be in worse condition after the cleaning than before, he said.Brodar has cleaning contractors work in both directions from a single manhole during a turnaround, while limiting the scope of work to a reasonable length of pipeline for each outage. Work during the next outage then will begin at an adjacent manhole. Because of the difficulty of access he limits the amount of maintenance work to what is reasonably achievable in the outage period.
Brodar prefers cleaning pipe interiors with ultra-high-pressure water jetting (above 25,000 psig) or low-pressure water cleaning (between 3500 and 5000 psig), depending upon the size and scope of the job. Both types of water cleaning require the use of a rotating tip for production rates and chemical decontamination to remove chlorides and sulfates.
Once corrosion has started, it will continue to attack the same locations on a steel pipe’s interior, no matter how many times it is cleaned and repainted, said Brodar. That is because interior corrosion is caused by nonvisible soluble salts, which react with the steel, oxidizing it.
Nonvisible soluble salts are primarily chlorides and sulfates. The chloride buildup occurs in circ-water systems because the water becomes concentrated over many cycles. Sulfates (SO4) build up because sulfuric acid (H2SO4) is added to control the pH of the circulating water. These salts are driven to the anode—the steel pipe—promoting corrosion.
This surface-contamination phenomenon is the largest problem facing paint in the 21st century, said Brodar, because we can no longer use lead-based paints for corrosion protection. The lead stops the corrosion induced by nonvisible salts by bonding with chloride.
Today’s immersion paints are barrier coatings, not inhibitive coatings. Instead of relying on the paint to inhibit corrosion, engineers now must ensure complete removal of the salts before painting. It’s more work, but it can be done.
Blast cleaning to white metal clears the obvious corrosion, but it also embeds many of the contaminants in the steel, Brodar said. If that occurs, flash rusting is inevitable, even after dry blasting. High humidity alone will not trigger flash rusting on an uncontaminated blasted surface; direct water contact is necessary for that to happen. But a blast-cleaned surface with trace contamination of soluble ions will flash-rust because the ions are hygroscopic, meaning they absorb moisture from the air.
Brodar prefers to pressure-wash with Chlor-Rid, a chemical decontaminant that removes soluble salts, using a zero-degree rotating tip that sprays a thin jet of water in a circle at 3500 psig. Pressure-washing is often done with a fan tip, which cleans broadly but not deeply into pits, he said. It is effective for the still coated, uncorroded surfaces, but it leaves traces of the soluble salts in the pits, and rust will reappear, often within minutes. When the rotating jet, on the other hand, is moved slowly over the surface, it cleans deeply, leaving a characteristic pattern, and the salts are completely removed (Fig 8).
Brodar tests steel for contamination with a small sheet of lab paper or filter paper impregnated with a solution of 4 milligrams of potassium ferrocyanide in 100 mg of water, which he prepares himself. It dries with a bright yellow color. He wets the spot to be tested, then presses the paper on it. If it turns blue, the surface is contaminated with chloride or sulfate (Fig 9). Tests after cleaning reveal spots where the salts remain (Fig 10). In that case, cleaning continues until there is no blue on the paper.



People who can do quality work inspecting circulating-water pipes are a subset of qualified powerplant inspectors, said Brodar. They have to be able to handle confined spaces, heights, and darkness without fear. The old rule is, “If you’re going to a powerplant, you should have a flashlight,” he said. “If you’re going someplace dark in a powerplant, you should have three or four flashlights.” His inspectors now use LED lights affixed to hard hats, which Brodar calls “a phenomenal improvement over the old stuff” (Fig 13).Removing nonvisible soluble salts is more important than removing all rust, Brodar said (Fig 11). A steel surface with light-brown rust can be painted over as long as there are no darker spots (Fig 12).




For steel pipe, if the diameter-to-thickness ratio is greater than 108 (D/t > 108), the pipe is susceptible to collapse from either vacuum or external load. Wall thickness in steel pipe determines its structural strength, of course, and one of the best defenses against failure caused by corrosion is thicker pipe walls. But thicker walls cost more money, so the economic reality is to design and install steel pipe just thick enough to handle the pressure. Brodar’s approach to inspecting pipe is thorough, but pragmatic. He doesn’t look for problems in the finish or coating where they are not readily visible because he figures that coating adhesion is thoroughly tested in service (Fig 14). Besides, he said, adhesion is not the main thing; after a system has been in service, the problem areas are identified: they are corroding (Fig 15). The key is to clean up chlorides and sulfates at the corroded sites, because they are the cause of corrosion. In FRP or fiberglass pipe, he looks for bulging, cracks, or chips. If you push on a spot and find it soft, he said, you have a mechanical-strength problem and you will need a fiberglass expert to solve it.
However, it is not necessary to double the steel-plate thickness to double its time-to-failure by pitting. Applying the “cubed-root law” when designing pipes determines how much increased pipe thickness can extend service life, Brodar said. If a pit would fully penetrate a pipe wall with thickness t in five years, then doubling the wall thickness would extend the service life 2[3]t x 5 (8t x 5) years for an expected life of 40t years. Increasing the wall thickness 25% would almost double the expected service life 1.25[3]t x 5 (1.95t x 5) years to 10 years.
The failure at Southwestern Public Service’s Harrington Station Unit 3, mentioned at the start of this article, illustrates these calculations. The failure occurred in a mastic-coated, unlined, 32-in. pipe at the top of the inspection riser buried under a 6- to 8-in. concrete slab and a couple of feet deep in compacted sand.
The pipe, in service since 1980, was supposed to be 0.375-in. wall, which would have given it a diameter-to-thickness ratio of 85.33, but it was found to be actually 0.1875 in. With a D/t ratio of 170.67, the pipe’s 30-yr service life without collapse is remarkable in itself. The pipe failed because its mastic coating failed, but “had it been design spec, it probably would have lasted a few more years,” said Xcel’s David Low.
The cubed-root law for pitting pegs that extension at approximately 2[3]t x 30 or 240 years. The Harrington failure was caused by general corrosion, not pitting, so the cubed-root formula is not likely to give an accurate prediction of increased time to failure, Brodar said, but it supports the opinion that the originally specified thickness would not have failed in just 30 years.
Xcel Energy is taking the lesson of its circulating-water line failure to heart. In the month following the break, Houston-based Intertek Aptech did a pulsed eddy current inspection of the system, and David Low will have the underground lines coated with 0.25-in. of polymer during the next planned outage.
Inspectors also accessed the interior of the failed riser via ladders for visual inspection and found the interior rough. The pipe is not normally accessible from the outside, so the company did not often inspect the 32-in. line, and the interior was not previously scraped and painted. That maintenance was done as well. “It’s a heads-up for all our plants from Minnesota to Colorado to New Mexico,” Low said. CCJ
1. What causes corrosion?
Man’s struggle with nature is so much a fact of life that it is a cliché. Overlooked is the fact that Mother Nature is a sore loser. She may retreat after a battle, but she never gives up on the war. That’s the reality behind the phenomenon known as corrosion.
A steel pipe is the product of manufacturing processes that use energy to convert iron ore into steel. Nature yields the ore to the miner, but once the pipe is fabricated, chemicals in its environment react with the material, and it begins to return to its natural condition as iron ore. Unchecked, the process of corrosion will end in the destruction of the steel. The energy that was stored in the steel during manufacture is released as electric energy that accompanies the chemical changes. Thus, corrosion is an electrochemical process, a combination of chemical reaction and electric energy.
Corrosion occurs in an electric circuit known as a galvanic cell (Fig A), which consists of a cathode, an anode, a conductive path between them, and an electrolyte. An automobile battery is a galvanic cell in which the electrochemical reaction is harnessed to produce electricity: The negative terminal, a metal, is the cathode; the positive terminal, a different metal, is the anode, and the battery acid is the electrolyte.
When the anode reacts with the electrolyte, it releases particles of its metal in the form of positively charged ions, leaving behind negatively charged free electrons. The ions flow through the electrolyte to the cathode, building a net-positive charge. The difference in potential drives the free electrons from anode to cathode, generating electric current.
In the automobile battery, the electrolyte is dilute sulfuric acid, but other materials can also be electrolytes. Water is the most common. For a buried steel pipe, the soil in which it is embedded plays the role of the electrolyte. If there is a moisture difference or two different soil types are in contact with the pipe, anodes and cathodes will form on the pipe. In that case, corrosion occurs because the pipe is in contact with two dissimilar electrodes, and the pipe itself acts as the anode, the cathode, and the conductor.
“Corrosion is caused by differences,” said John Brodar. In a powerplant’s circulating-water cooling system, the pipes are made of coated steel and they come in contact with other materials, including soil, concrete, steel encased in concrete, copper, and stainless steel on the exterior side. On the interior, the steel material may be in contact with copper or brass condenser materials, stainless steel, and chemicals in the cooling water. The potential for corrosion exists wherever two dissimilar metals are joined in a galvanic couple.
Metals are sorted in the galvanic series (table) according to the difference in potential between them, measured in volts. At the anodic end, with the highest negative charge, are magnesium, zinc, and galvanized steel. At the cathodic end, with the highest positive charge, are platinum, gold, and graphite (carbon). The anodic end of the series is called the active end and the cathodic the noble or passive end because the the anode releases ions and the cathode receives them. Most important is the fact that the anode corrodes while the corrosion resistance of the cathode increases.
Corrosion depletes or corrodes the anode, which is the metal closer to the active end of any galvanic couple in the series. Iron and steel carry a lower negative charge than zinc, so pairing a steel pipe in an electrolyte with a zinc anode will protect the pipe while allowing the zinc anode to corrode (Fig B). The rate of corrosion in the anode can be reduced if the anode and cathode are close neighbors in the galvanic series, with only a small potential difference between them.
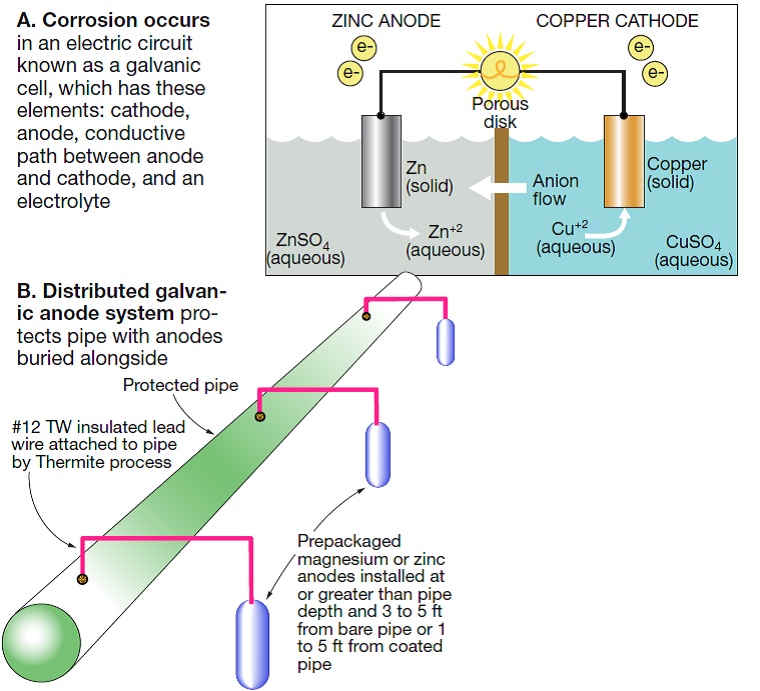

2. Protecting the pipe you can’t see
Corrosion occurs when ions flow from an anode into the electrolyte. Every corrosion cell has four elements: an anode, a cathode, an electrolyte, and a metallic path (Sidebar 1). Cathodic protection does not stop corrosion; it transfers corrosion to a specific structure known as a cathodic protection anode. Cathodic protection works by changing the protected structure (the circulating-water line) from being the natural anode (corroding) to a cathode free of corrosion.
This change from anode to cathode is caused by direct-current flow. With a directed electric current flow, from the cathodic protection anode to the protected structure in the electrolyte, corrosion on the circulating-water line will be reduced and, with sufficient current, it will be stopped entirely.
Cathodic protection works by connecting an anodic metal to the steel pipe and allowing a direct current to flow between them. The effectiveness of cathodic protection is measured by the potential (voltage) of the protected structure compared to a reference electrode. The pipe is one-half of a galvanic cell; a “reference cell” is the other half. When they are connected through a voltmeter, they make a complete battery, whose voltage can be measured.
Electric potential is idiosyncratic: Every galvanic couple has its own, so the National Association of Corrosion Engineers (NACE) criterion for protection is that if steel is more negative than –0.850 V relative to a copper/copper sulfate cell, it is completely protected and will not corrode. The Cu/CuSO4 cell thus is the reference cell for the NACE standard, a yardstick for measuring corrosion protection that can be applied to other couples.
In a cathodic protection system, an anode of zinc typically is buried near the steel pipe and backfilled with bentonite clay, calcium sulfate, and sodium sulfate. The potential is checked by connecting a lead from the pipe to the red wire on the voltmeter and a lead from the reference cell to the black wire. If the potential of the protected structure is more negative than –0.850 V relative to the Cu/CuSO4 reference electrode, it will not corrode.
Coatings and cathodic protection work synergistically. The coating does most of the corrosion control, and cathodic protection provides the corrosion protection at the flaws in the coating. Without cathodic protection, the structure will corrode at the flaw. It is cheaper to protect most of the surface of the pipe with coating, which reduces the amount of current required for cathodic protection. Corrosion will be halted as long as the cathodic protection system is maintained such that the protected structure potential is more negative than –0.850 V.
The anode’s sole function is to sacrifice itself and provide protective current. Over time, it will corrode and the dc flow will have to be increased to maintain protection. Periodic inspections by close-interval survey can confirm the protection by measuring the potential and confirming it meets the criteria over the length of the pipe. A close-interval survey normally checks protection about every five feet.
Generally, when a lot of current is required in a single location it is more economical to use an impressed-current system instead of galvanic anode. The impressed-current system supplies direct current from an outside source called a transformer rectifier, but the description of the sacrificial-anode system suffices to explain the principles behind this form of cathodic protection.



