Accurately measuring iron key to controlling cycle chemistry
Doing right by your plant’s steam/water cycle chemistry just got a little easier. Any added expense is minimal or negligible, according to at least one expert, although one user notes the cost of the program is “significant” but well worth it.
Document helps justify cost for improvement to management
Hayden Henderson, station chemist and environmental manager at NewGen Power’s Kwinana Pty Ltd 320-MW combined-cycle plant south of Perth, Australia, has put the IAPWS document to work. He notes many benefits, but perhaps the top one is the document itself: “It made it easy to justify the cost of improving our systems to site management.”
While the overall cost of implementing in-house iron monitoring with IAPWS compliant sampling was “significant,” NewGen feels the benefits of accurately monitoring corrosion products will substantially outweigh those costs in the long term.
The sampling program was implemented over the past 12 months. The plant purchased a new UV-Vis spectrometer and a sample digestion block. Samples are digested with thioglycolic acid and analyzed using the Ferrozine method. In addition, backpressure regulating valves were installed for all the sample points to allow stable flow to the instrument and grab-sample points. Henderson notes that an additional sample point on the low-pressure (LP) economizer will be installed within the next year.
When asked what could be improved within the document, Henderson’s comment was “expanded commentary on the fact that the desired flow velocities cannot be achieved for all parts of the system, especially the HRSG LP evaporator.”
He further noted that the most helpful aspects of the document are the emphasis on correct sampling, standardizing results, and the discussion of alternative analytical methods and sample digestion methods. “It is not feasible to have an ICP-MS on site for a plant of this size,” he said.
Today, Henderson can complete a sampling test with five samples from the same sample point, one after the other, and the results will agree within 2%, lending strong confidence that the results are accurate and represent actual conditions in the water/steam cycle.
Previously, using several different methods, including grab samples analyzed externally by an inductively coupled plasma mass spectrometer (ICP-MS) and filter samples analyzed externally by ICP-MS, duplicate samples often disagreed by 10% at best, and results generally were erratic. This made it difficult to determine the success of deliberate cycle chemistry changes in decreasing corrosion product transport.
The International Assn for the Properties of Water and Steam (IAPWS) has issued a technical guidance document (TGD), Corrosion Product Sampling and Analysis for Fossil and Combined Cycle Plants, which, for the first time, supports a common baseline for plants worldwide to validate results and maintain low-risk cycle chemistry. Major HRSG manufacturers were involved crafting the document, so there are few worries of getting cross with your vendor.
IAPWS member David Addison, principal consultant at Thermal Chemistry Ltd., puts it simply: “You’re not in control of your cycle chemistry if you are not accurately measuring total iron and copper content.” The problem today, in blunt terms, is that most combined-cycle owner/operators either don’t measure corrosion products or they, or their service providers, are employing incorrect methods. It’s no wonder: Some facilities no longer have a chemist onsite, proper laboratory equipment, or properly trained operators—if they ever did.
The new document helps you attain best world practice: For combined cycles with optimized cycle chemistry, the optimal total iron level specified in IAPWS TGDs is
Importantly, for today’s razor-thin combined-cycle staffs, this isn’t something you have to do constantly, or even weekly. “You need to devote a few weeks to establishing the baseline condition, and then one week or so of sampling once or twice a year, unless something occurs to disrupt the baseline condition, in which case it needs to be re-established,” notes Addison.
The sampling ports and equipment are usually in place. Typically, less than $20,000 is required to purchase the equipment. If plant staff does not handle this task, you should review the TGD with your chemical sampling and analytics vendor and make sure it either adheres to its guidelines or offers a good explanation why not.
“As plants use the TGD and produce robust corrosion-products data, the industry will be able to standardize, providing a benchmark for individual plants to compare and validate their results and impacts and to improve their cycle chemistry,” Addison comments.
Plants often have turbidity and particle-counter monitors, but these are proxy measurements at best for total corrosion products. They are unsuitable as a primary method, though they can be validated through the TGD. “Most turbidity meters are designed for drinking-water service, not powerplants,” decries Addison. Regarding the latter, the chemist says, site-specific validation generally is not conducted.
In broad strokes, the document covers the following:
• Highlights problems with physical sampling of corrosion products in flowing water and steam, and indicates how to avoid such problems.
• Suggests key sampling locations for different systems (for example, combined-cycle facilities with air-cooled condensers or traditional shell-and-tube surface condensers) and feedwater treatment regimes (for example, AVT or other).
• Outlines, with diagrams, an optimum system for representative sampling.
• Illustrates advantages and disadvantages with all existing, common sampling and analytical methods.
• Suggests a level of quality control to ensure reliability, accuracy, etc.
One common problem is that plants tend to sample when it is convenient to them rather than what is best for accuracy and reliability. One general criterion is that sampling be conducted at some reference conditions, preferably when operating above 80% load with the plant stable for more than two hours. This, of course, highlights one of the difficulties as many combined-cycle facilities frequently are ramped up and down in load. This is all part of establishing a clear baseline for the plant and building a reference baseline for similar plant groups worldwide.
Another common plant issue is the use of a spectrophotometer with no chemical digestion (meaning only the soluble component is detected, and not the particulate; hence, the results are lower than they actually are) of the samples prior to analysis. Digestion of particulate corrosion products requires the use of a strong acid, heat, and time. “The sensitivity of the analytical technique must be matched to the level of corrosion products in the system,” stresses Addison.
Finally, choosing sub-optimal sampling locations is common. The optimum location is from a stream of moderate-to-high velocity downstream of, but as close as possible to, the component or area of a circuit under surveillance.
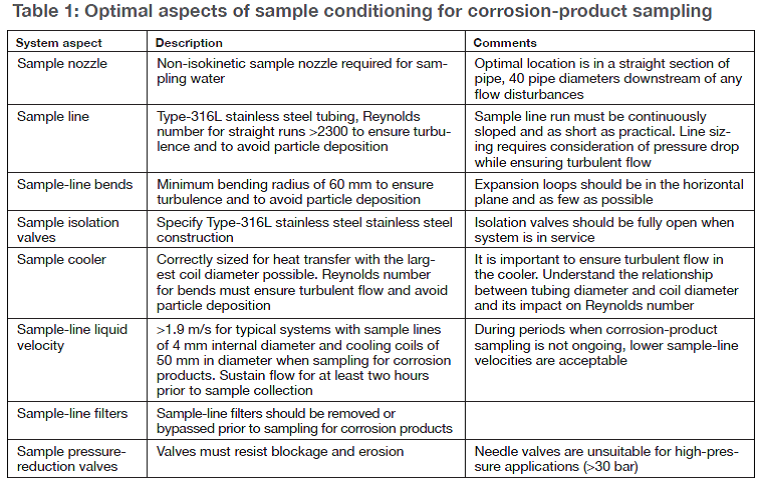
Table 1 reviews the optimal aspects of sampling. Another very helpful table summarizes all the analytical techniques for iron and copper. You can check what you are doing to the critique provided. The important point is that, while many techniques are reviewed, only certain ones listed in Table 2 are considered suitable for total corrosion product levels in the ranges commonly found in HRSGs.
If your current practice is not aligned, then download a copy of the TGD from the IAPWS website. It’s FREE, clear, and easy to digest. As a complement to your inspection programs (and in conjunction with other guidelines available at the IAPWS site), you’ll enjoy greater peace of mind that you are keeping your plant and workers safe from the slow deteriorating and/or sudden catastrophic effects of corrosion and deposition. CCJ
Table 2: Analytical methods for determining the concentration of iron in feedwater
Corrosion Product Sampling and Analysis for Fossil and Combined Cycle Plants is one of the most important technical guidance documents (TGD) published to date by the International Assn for the Properties of Water and Steam (IAPWS) for owner/operators of combined-cycle plants.
The long-term health of the Rankine cycle demands that operators adhere to the “Rule of Two and Five” championed by Dr Barry Dooley of Structural Integrity Associates Inc, the IAPWS executive secretary. It means that combined cycles with optimized water chemistry should restrict the total iron level in feedwater to <2 µg/L and to <5 µg/L in HRSG steam drums.
Table 6 on pages 26 to 29 of the TGD cited summarizes the analytical methods recognized for determining concentration of iron in feedwater and drum water. This document and others can be accessed at www.iapws.org and downloaded as pdf files.
Information contained in the table is of greatest value to the person responsible for cycle chemistry at your plant. However, it is important for operations personnel to familiarize themselves with the tests available, and their limitations, to enable better decision-making. The editors have extracted that content from the table for the CliffsNotes version below. Several tests included in Table 6 have been omitted from the following summary because their detection limits are less than the recommended total iron values in the rule stated.
Babcock & Wilcox Membrane Filter Comparison Charts. Perhaps the analytical method in this list most familiar to operations personnel, the B&W procedure is not suited for optimizing cycle chemistry in an operating plant. However, it is useful for indicating the presence of particulate iron during plant commissioning activities.
The test involves filtering a 1 liter sample through a 0.45-µm filter under vacuum. Filter residue is compared visually to a reference chart to guesstimate iron concentrations between 15 and 250 µg/kg. By way of background, the method was developed for the commissioning of once-through fossil-fired boilers.
Ultraviolet/Visible (UV-Vis) Spectroscopy after sample digestion. This colorimetric method is based on Ferrozine reaction after a suitable digestion step where particulate iron oxides are converted to Fe ions.
Important to note is that most commercially available portable UV-Vis spectrophotometers are supplied with 1-cm cells with a detection limit of 9 µg/kg—unsuitable for powerplant use. Be sure to specify a 5-cm cell, which has an acceptable detection limit of 2 µg/kg. Also worth noting is that the optimum digestion method for particulate iron is to add thioglycolic acid to the sample and heat to 90C.
Graphite Furnace Atomic Absorption (GF-AA) Spectroscopy after sample digestion to convert particulate iron oxides to Fe ions. Method is suitable for total iron corrosion products because the detection limit of 0.3 µg/kg is less than the optimum feedwater total iron value. Particulate iron digestion by way of boiling nitric acid is recommended.
Inductively Coupled Plasma Mass Spectroscopy (ICP-MS) involves sample digestion to convert particulate iron oxides to Fe ions using boiling nitric acid followed by detection and quantification via mass spectroscopy. Method is suitable for total iron corrosion products because the detection limit of 1 µg/kg is less than the optimum feedwater total iron value.
Corrosion Product Sampler. A sample is collected on a 0.45-µm filter (see B&W method above) followed by an ion-exchange filter at the sample-point operating pressure after sample cooling. Sample total filtered liquid volume recorded. Next, filters are removed and digested—generally using thioglycolic acid at 90C or boiling nitric acid. Total mass of iron on and in the filters is determined using a suitable dissolved-iron analysis method, such as UV-Vis (ferrozine), ICP-MS, or GF-AA. Finally, the total mass of iron is divided by total sample volume to give average total iron concentration for the sampling period. Sample collection requirements: Connection point downstream of the sample cooler, high-pressure sample filter holder, and high-accuracy totalizing flowmeter.



