By Peter Dufresne Jr, Matthew G Hobbs, Glen MacInnis, EPT
Nearly 10 years ago, GE reported that approximately one in three large industrial gas turbines showed signs of oil varnishing. Because this condition affects the availability and reliability of GTs, the OEM recommended the use of varnish removal systems. Despite the recommendation, varnish-related turbine outages remain a significant issue for the industry.
The primary reason varnish is an ongoing problem: The mechanism by which varnishing occurs is poorly understood by many turbine owner/operators. Thus, strategies aimed at correcting or mitigating varnish-related problems often are misdirected, resulting in less than ideal outcomes. The goal of this article, based on a presentation by the principal author at the 2013 Conference of the Frame 6 Users Group, is to improve understanding of varnish by discussing its specific cause and how various mitigation alternatives work to minimize operational impacts.
Varnish formation
 Lubricant varnish generally is defined as a thin, hard, lustrous, oil-insoluble deposit composed primarily of organic residue. It is most readily defined by color intensity and is not easily removed by wiping.
Lubricant varnish generally is defined as a thin, hard, lustrous, oil-insoluble deposit composed primarily of organic residue. It is most readily defined by color intensity and is not easily removed by wiping.
While this definition provides an adequate description of varnish at the end of its life cycle (Fig 1), it must be expanded as follows to account for the remainder of the varnish cycle: Varnish begins its life as a soluble degradation product before converting to an insoluble particulate form. The process responsible for the deposition of particulate varnish is reversible.
This expanded definition reveals that varnish is a shape-shifter; it can be insoluble (conventionally recognized particulate form) or dissolved (soluble) in the fluid. An understanding of lubricant solvency is the key to understanding the mechanism by which varnish deposits are formed and, more importantly, the mechanism by which they can be removed.
Lubricant solvency
Under normal operating conditions, turbine lubricants are subjected to oxidation, which produces polar molecules (varnish precursors) from non-polar ones (lubricant mineral-oil base stocks). These polar species represent the starting point of the varnish life cycle. As a result, lubricants in service are a complex combination of base stocks, additives, and contaminants.
A lubricant’s solvency is defined as its ability to dissolve these distinct components. Everything in the oil has a finite solubility, which is affected by numerous variables (molecular polarity, contaminant levels, temperature, etc). This solubility determines if a particular molecule is soluble in the fluid or if it will precipitate from the fluid to form a potentially damaging deposit.
When the solubility of a molecule is low, the lubricant cannot dissolve it and will actively release it, producing deposits. However, when the solubility of a molecule is high, the lubricant will have a high capacity to dissolve it, avoiding the formation of varnish deposits.
Factors affecting lubricant solvency
The following factors play a major role in determining the solubility of varnish precursors in lubricants:
Molecular polarity. The polarity of a molecule refers to the distribution of positive and negative charges within it. In some molecules, these charges are well separated (like the poles of a bar magnet); such molecules are said to be polar. In others, there is little or no separation of charge; these molecules are said to be non-polar.
Molecular polarity is not simply black and white. The polarity scale incorporates shades of gray. Because polarity depends on the specific structure of every molecule, it is possible for one polar molecule to be more, or less, polar than another. The corollary for non-polar molecules is also true. The most basic axiom of solvency is that “like dissolves like.”
This accounts for the fact that polar alcohol will dissolve fully in polar water while polar water will not dissolve in non-polar mineral oil. Although the varnish precursors produced by oxidative degradation of mineral oil base stocks are polar, they are much less so than water. Consequently, these somewhat polar degradation products have some finite solubility in a lubricant’s non-polar mineral-oil matrix. Degradation products that are more polar will be correspondingly less soluble.
Contaminant levels. A lubricant has a finite capacity to dissolve other molecules (additives, contaminants, varnish precursors, etc). As the oil degrades and oxidation products accumulate, the solvency of the fluid decreases accordingly. Beyond a certain point (known as the saturation point), the fluid can no longer dissolve additional varnish precursors formed by continuing oxidation and varnish will begin to precipitate from solution in the solid form.
Temperature. Oil temperature directly affects the solubilities of all the species dissolved within it. As temperature decreases, so does the solubility of varnish and its precursors. In the sugar industry, hot solutions of table sugar are cooled to decrease the sugar’s solubility. As the sugar’s solubility falls, crystalline table sugar is deposited from the solution. This same process is responsible for the precipitation of varnish deposits in cooler regions of a turbine’s lubricant circulation system. Because metals are more polar than the lubricant’s base stock, the precipitated polar varnishes prefer to adhere to the metal and form potentially damaging deposits. When the level of varnish precursors in a lubricant is at (or near) the fluid’s saturation point, varnishing in cooler regions is very likely to occur.
The varnish cycle
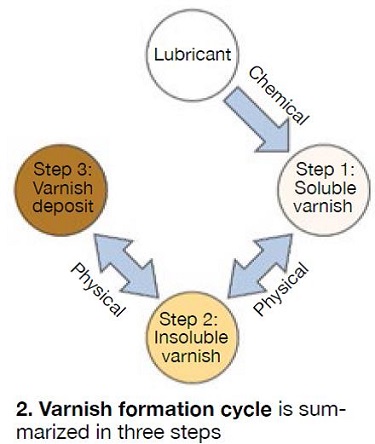 The typical varnish formation cycle in a gas turbine involves these three steps (Fig 2):
The typical varnish formation cycle in a gas turbine involves these three steps (Fig 2):
1. Oxidation is a chemical reaction between the lubricant base stock and oxygen present in the air surrounding it. Oxidation is unavoidable and begins to take place the instant that a new fluid is exposed to air for the first time, regardless of whether or not the fluid is put into service. Like all other chemical reactions, the rate of oxidation is bound by the Arrhenius equation, which states that the rate of reaction will double for every 10-deg-C (18 deg F) increase in temperature.
Once a new fluid is put in service, it is exposed to higher temperatures and experiences a concomitant increase in the rate at which it oxidizes. Even when operating temperatures are a typical 125F, bearings may reach temperatures in excess of 300F; the rate of oxidation at the bearing in this instance will be more than 1000 times greater than that in the cooler regions of the system. As a result, oxidation typically occurs wherever hot spots are found.
Oxidation products build up in the lubricant over time, but remain dissolved at operating temperatures unless they exceed the fluid’s saturation point.
2. As the oil moves from hotter regions within the system to cooler ones (hydraulic lines supplying high-pressure oil to engine geometry control, for instance), the fluid temperature falls and the solubility of any varnish precursors present decreases. These precursors begin to precipitate from solution in the form of particulates. Like water freezing to form ice, this precipitation of varnish is a physical change and not a chemical reaction.
3. Once formed, varnish particles agglomerate and form deposits, preferentially coating metal surfaces. These deposits are often the cause of unit trips or fail-to-start conditions. Like precipitation in Step 2 above, agglomeration and deposition are physical changes. [RM]
This model of varnish formation is widely accepted and reasonably well understood. Less well-understood is the fact that once varnish deposits form, they can be reabsorbed, if the solvency of the lubricant is increased. While the chemical changes that lead to the formation of varnish precursors (Step 1) are irreversible, the physical changes (Steps 2 and 3) which lead to the formation of varnish deposits are reversible. Successful varnish mitigation strategies use this fact to their advantage.
Testing for varnish
As a result of the potential for costly turbine downtime associated with varnishing, it is imperative that a lubricant’s propensity to form varnish deposits be determined. Most turbine users test their lubricants for varnish potential using widely adopted techniques including QSA® (quantitative spectrophotometric analysis) and the standardized test MPC (membrane patch colorimetry, ASTM 7843). Proprietary (non-standardized) varnish test methods are not recommended, as they are not widely used and cannot be readily corroborated. Other collaborative analyses, like patch weight, may be helpful in substantiating oil health.
Both of the above varnish measurement methods can produce results which vary significantly depending upon the length of time during which the oil sample was “aged.” Indeed, longer sample aging periods produce higher MPC values, suggesting that degradation of lubricants continues in the sample bottle. For this reason, the ASTM MPC method suggests all samples be incubated at room temperature for 72 hours after being heated to 140F for 24 hours. This well-defined and standardized aging time has provided inter-laboratory consistency and improved repeatability.
Increasing MPC and QSA values during sample aging occur as a result of the continuing propagation of oxidation reactions that were likely initiated when the lubricant was in service. Oils that continue to degrade in a sample bottle will also continue to degrade in a lubricant reservoir. This highlights the necessity of using varnish removal equipment on a continuous basis. In the absence of varnish removal equipment, lube-oil reservoirs with accumulations of dissolved break-down products can continue to form varnish when the turbine is not operating.
Strategies to combat varnishing
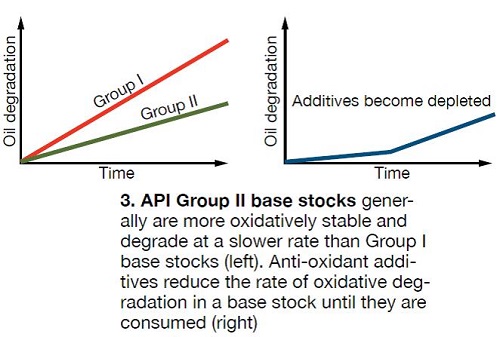
Most modern turbine lubricants are made with API (American Petroleum Institute) Group II mineral-oil base stocks, which contain an anti-oxidant additive package. The chemistry of Group II base stocks makes them more oxidatively stable than the traditional Group I base stocks that they generally have replaced in turbine-oil applications (Fig 3).
 Anti-oxidants usually are added to the lubricant as a built-in varnish mitigation strategy. These additives generally are comprised of two classes of chemicals: phenols and amines. Although both have anti-oxidant activity on their own, they function more efficiently in concert with one another. While the specific identities and amounts of the anti-oxidants employed varies with different lubricant formulations, the mechanism by which they enhance fluid lifetime remains the same.
Anti-oxidants usually are added to the lubricant as a built-in varnish mitigation strategy. These additives generally are comprised of two classes of chemicals: phenols and amines. Although both have anti-oxidant activity on their own, they function more efficiently in concert with one another. While the specific identities and amounts of the anti-oxidants employed varies with different lubricant formulations, the mechanism by which they enhance fluid lifetime remains the same.
These chemical additives are sacrificial and will oxidize more readily than the lube-oil base stock. As a result, the oxidation rate of the oil itself is decreased, while anti-oxidants are present. Unfortunately, both phenols and amines become consumed as they oxidize; the phenols tend to deplete more quickly. Once the additives are consumed, the rate of fluid degradation accelerates, returning to that of the non-additized base stock (Fig 3). Anti-oxidants limit the rate oxidative degradation and, therefore, delay varnishing, but they cannot prevent it.
Since anti-oxidant levels deplete continuously, it is important to monitor them to ensure that your lubricant is protected from excessive degradation. Methods for monitoring additive depletion include voltammetry (RULER testing ASTM 6971), Fourier transform infrared (FTIR) spectroscopy, and high-pressure liquid chromatography (HPLC). Regardless of the monitoring method employed, the fluid should be replaced when all anti-oxidant additives have been consumed.
While the anti-oxidant additives included in most turbine-oil formulations are an essential tool in the fight against varnish, as noted above, they can only limit oxidative degradation, not prevent it. When the lubricant inevitably oxidizes and varnish precursors are formed, varnish removal systems are necessary to prevent degradation products from accumulating to the point where varnishing occurs. There are two main types of varnish removal systems: those based upon the removal of suspended (insoluble) particles and those based upon the removal of soluble varnish and its precursors.
Suspended-particle removal systems. Depth filtration, Balanced Charge Agglomeration™, electrostatic oil cleaning, or combinations of these techniques are advanced forms of particulate removal. These techniques remove fine particulates that are suspended within the fluid, including insoluble varnish particles. As particulate removal technologies, these systems must wait for insoluble varnish particles to form before they can be of value.
Since solvency decreases at lower temperatures (favoring the formation of insolubles), the maximum benefit obtained using these systems is achieved when the turbine is not operating and the lubricant is at ambient temperatures. Suspended particle removal systems are, therefore, of more use when employed periodically, during outages; they are less effective when used continuously during turbine operation. When used in the manner described above, these systems are incapable of removing soluble varnish and its precursors.
In an effort to overcome this limitation and enable continuous use, oil coolers can be used on the inlets of these systems to accelerate the varnish formation cycle and precipitate insolubles from the lubricant immediately before it passes through the varnish removal system. This form of varnish removal is referred to as “temperature-induced varnish removal.”
However, the magnitude by which the oil can be cooled is limited: Cool oil is more viscous and difficult to pass through the particle removal systems. Because of this limitation, the oil cannot be cooled to the temperatures required for complete removal of all of the soluble varnish present. Result: The lubricant’s solvency is never improved to the point where varnish deposits already present elsewhere in the system can be re-dissolved into the fluid.
Moreover, the soluble varnish and soluble varnish precursors, which cannot be removed from the fluid, return to the turbine where they may plate out on metal surfaces. As more varnish is deposited, the lubricant becomes perpetually saturated and further varnish removal is impaired. As varnish continues to build up, the suspended particle removal system often will be unable to keep up.
Soluble varnish removal (SVR™) systems use specialized Ion Charge Bonding (ICB™) ion-exchange resins that contain billions of polar sites capable of adsorbing soluble varnish and its precursors. This adsorption relies on a preferential molecular interaction between the polar varnish molecules and the polar sites present within the resin. Just as polar varnish prefers to coat polar metal surfaces, so too it prefers to adsorb on the polar sites of the ICB resin.
Conventional ion-exchange resins function by exchanging one chemical for another. Unlike these resins, which exchange one contaminant for another, ICB resins are engineered to adsorb the entire contaminant without returning any others to the fluid.
A key benefit of the ICB adsorption principle is that harmful oxidation products can be removed at any operating temperature, meaning that SVR systems can be used continuously. The continuous removal of soluble varnish and its precursors ensures that degradation products do not accumulate in the lubricant, eliminating the risk of varnish formation during normal turbine shut down cycles. Moreover, the continuous removal of soluble varnish produces a lubricant with extremely high solvency.
Since the physical changes that resulted in the formation of insoluble varnish particles and deposits are reversible, the high solvency of the SVR-treated lubricant, forces insoluble varnish already present on turbine surfaces back into the soluble varnish form where they can be adsorbed and removed. With all the remaining oxidation byproducts removed, the varnish formation cycle is completely stopped.
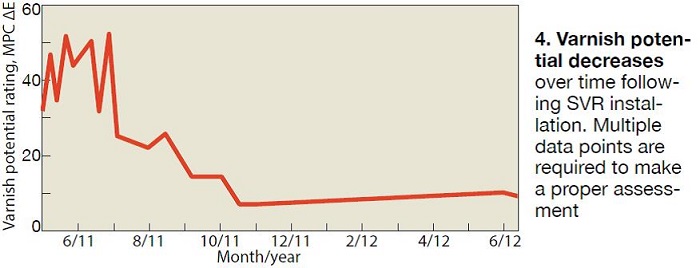
Fig 4 illustrates the usual trend in MPC values for one year following the installation of an SVR system. There are two distinct phases in this example. The first is the “restoration” or “clean-up” phase. The second is the “stability phase.” As SVR treatment is initiated and the restoration phase begins, the MPC value increases initially. For many users, an initial increase in the fluid’s varnish potential following the installation of an SVR is concerning; however, such an increase is typical and, indeed, demonstrates that the SVR is accomplishing its goal.
As previously insoluble varnish deposits are cleaned by the lubricant, which now has the solvency required to return them to the soluble state, the level of soluble varnish increases resulting in higher MPC measurements. This restoration phase typically lasts for three or four months, but longer durations are possible, depending upon the level of contamination present.
When a system is relatively clean and contains few or no varnish deposits, the fluid’s varnish potential begins to drop immediately following SVR use. Once MPC values decrease below 10, the lubricant enters the stability phase. In this state, the oil contains minimal levels of oxidation products/varnish precursors and has a high solvency. Turbine operation under these conditions is ideal, as the high lubricant solvency and low concentrations of soluble varnish precursors prevent varnish from forming under the variable operating temperature and pressures conditions employed in most turbines.
Soluble varnish removal
Varnish particles and deposits are created from reversible physical changes that begin with soluble oxidation products and end with insoluble deposits. For these changes to be reversible, the chemistry of the deposits has to be similar to the chemistry of the lubricant from which the deposits originated. Normally, once fluid solvency has been increased (by removing soluble varnish at normal operating temperature), deposits will simply dissolve back into the fluid and be removed.
However, when one lubricant is replaced by another type, it can impair the ability of deposits or remaining varnish particles to return to their soluble form. An immediate oil change can, therefore, result in significant amounts of varnish being left on turbine surfaces. For this reason, old reservoirs should be cleaned prior to oil changes. The ICB process can be used to restore fluid solvency, allowing deposits created by the lubricant to return to their soluble form and be removed. In this manner, the reservoir can be cleaned and readied for new oil without ever having to drain it. CCJ
About the EPT authors
Peter Dufresne Jr, (pdufresne@cleanoil.com), director, and his father, Peter Sr, have championed the use of resin-based technology for lubricant purification for years. Matthew G Hobbs (mhobbs@cleanoil.com), senior chemist, has a PhD in synthetic chemistry. Prior to joining EPT, he was GM of a national oil analysis laboratory. Glen MacInnis (gmacinnis@cleanoil.com) has a master’s of science in chemistry and previously managed R&D at EPT.