By Wes Byrne, consultant, membrane technologies, U.S. Water
Pure water does not exist in nature. All natural waters contain varying amounts of dissolved and suspended matter. Osmosis is the process in which a solvent (water, for example) flows through a semi-permeable membrane from a less concentrated solution to one with a higher concentration. This normal osmotic flow can be reversed (reverse osmosis) by applying hydraulic pressure to the more concentrated (contaminated) solution to produce purified water.
There is no perfect semipermeable membrane. A small amount of dissolved salt is also able to diffuse through, but this results in relatively low concentrations compared to the feedwater values.
The benefits of reverse osmosis (RO) technology should be well understood in water treatment for power generation, particularly because of its potential to reduce O&M expenses. For most sources of water, RO will be the least expensive way to remove dissolved salts.
The term total dissolved solids (TDS) refers to these inorganic salts with some small amounts of organic matter, present in solution. The salts exist as cations (mostly calcium, magnesium, sodium, and potassium) and anions (mostly bicarbonate, chloride, sulfate, and nitrate). These positively and negatively charged ions can pass electrical flow, thus determining the conductivity of the water as a measurement of its TDS concentration. Pure water is a poor conductor of electricity.
 For plants originally built using only ion exchange, adding RO can reduce chemical regeneration requirements by a factor of 20 or more. Complete removal of regenerable systems might even be considered.
For plants originally built using only ion exchange, adding RO can reduce chemical regeneration requirements by a factor of 20 or more. Complete removal of regenerable systems might even be considered.
With RO upstream removing the bulk of the dissolved salts, the polishing ion-exchange systems might be economically replaced with service demineralizer beds that are chemically regenerated by an offsite water service company, or they might be replaced by electrodeionization. EDI units use electricity to continuously regenerate their resins.
Some new and existing plants are now being required to remove dissolved salts from their wastewater streams prior to discharge. RO may perform this role so well that it may even be possible to re-use the water within the plant. The concentrated salt stream remaining after RO treatment might then be more economically hauled to a region that can better handle the environmental effects, or it could be evaporated or discarded in some other manner. The political and regulatory advantages of becoming a zero-liquid discharge (ZLD) facility can offset part of the capital and operating costs.
But the superior economics of RO operation are only achievable if the system and its upstream treatment components are correctly designed, operated, and maintained.
Analysis
Pulling a water sample for laboratory analysis is a good start in preparing an RO design. A comprehensive analysis provides data on the metals in the water (for example, iron, manganese, and aluminum), the dissolved salts (cations and anions), the water pH (acidity), and possibly the inorganic total suspended solids (TSS). A measurement of the total organic carbon (TOC) will often correlate with the potential for biological activity (Table 1).
A TSS analysis reveals the concentration of filterable solids in the water. The concentration of dissolved metals, such as iron, will change in the water sample as they react with oxygen introduced by contact with air. This will cause some of the metals to oxidize and become insoluble. The metals that stay suspended in the water may cause the TSS value to increase significantly with many well-water sources.
Biological fouling solids will not be well represented in the TSS results. The mass of these solids will typically become negligible when the TSS filter is dried prior to weighing for results. The water could be tested for its silt density index (SDI) if the metals are first separated out of the sample. This test will be highly sensitive to the ability of biological solids to coat and reduce the flow rate through its 0.45-micron test filter. Results will correlate with the fouling tendencies of a membrane system.
No analysis is perfect, and water quality can change over time. Even the characteristics of a well-water source will change if the well is relatively shallow.
Sampling methods affect results. Some concentrations will change between sample pull and analysis. Metals may attach to the container’s inner surface. Ammonia and carbon dioxide may degas or carbon dioxide may dissolve from exposure to air. Any of these changes will cause the water pH to change. An accurate water pH is best measured onsite.
Chemical suppliers can use a water analysis to predict how much purified water (permeate) the RO might safely separate from the source before the dissolved salts become too concentrated in the remaining water and form scale within the membrane elements (Table 2). The water analysis is also used in designing the RO system, both in projecting the purified water quality and in assessing any effect of the salts on system hydraulics.
Pilot study
An RO system and its pretreatment equipment designed solely on one water analysis may not be fully optimized for the fouling characteristics of the source. It might be oversized or, of greater concern, it might not be ideal for water that has a high membrane fouling potential. This can best be determined with a pilot study (Fig 1).
A well-designed pilot study will use components that have been scaled down but still offer the same type of media and use similar flow velocities and exposure times. The pilot RO should duplicate the permeate recovery, the permeate flux rate (that is, permeate flow per unit of membrane area) and concentrate stream vessel exit velocities, along with the scale inhibitor dosage and shutdown flush methods.
When the pretreatment methods are piloted along with the RO, the system operation can be adjusted to minimize the rate of RO membrane fouling, such as by modifying the permeate flux rate or the rate at which water passes across the membrane surface and through the membrane elements. With the right equipment choices and sizing, it might even be possible to eliminate membrane fouling, which could then dramatically reduce operating cost and maximize membrane life (Fig 2).
The choice of membrane might also be evaluated. With large systems, demonstrating that a low-fouling membrane element performs better than a standard element will help justify its higher cost. Low-energy elements might be evaluated for their potential to reduce pump size and associated power consumption.
 The pilot study also offers an opportunity to learn more specifically about what will foul the RO system. A membrane element from the pilot study might be pulled and autopsied. Analysis of the solids then makes it possible to choose cleaning solutions that are best suited for removing the particular fouling materials. The effectiveness of the solutions and cleaning methodology might then be verified with the pilot unit.
The pilot study also offers an opportunity to learn more specifically about what will foul the RO system. A membrane element from the pilot study might be pulled and autopsied. Analysis of the solids then makes it possible to choose cleaning solutions that are best suited for removing the particular fouling materials. The effectiveness of the solutions and cleaning methodology might then be verified with the pilot unit.
The longer the pilot system can be operated, the more information will be gained. A minimum of several months is recommended.
Upstream equipment
The success of a new RO membrane system is often directly related to its pretreatment. Piloting the upstream processes can be challenging in sizing these components for the pilot’s low flow rate.
The most important role played by pretreatment is protecting the RO from incompatible substances. With the polyamide thin-film RO membrane commonly used today, the biggest concern is removal or destruction of any chlorine or other potentially oxidative compounds. This membrane has very little tolerance to free chlorine (present in many municipal water sources), and is only slightly tolerant of chloramines (in other municipal water sources).
The two most common methods for breaking down chlorine are reducing-agent injection and activated carbon filtration. The most common reducing agent is sodium bisulfite (NaHSO3) which will react preferentially with free chlorine in breaking it down to the innocuous chloride ion.
Sodium bisulfite/sulfite injection systems can fail in ways that will degrade RO membrane elements if not quickly remedied. Examples: The day tank could run out of solution, or the injection pump could lose power or pump-head prime. The injection pump setting might provide insufficient chemical to handle the full range of chlorine concentrations, or might be set for such a low pulse speed that the chemical does not sufficiently mix with the feedwater.
The proper sodium bisulfite dosage should be injected any time that the RO inlet valve opens, even if this opening is for filling the RO or for flushing it out before a shutdown. There cannot be significant pipe length distance between the point of injection and the RO inlet valve, since this length will become fully chlorinated during shutdown by chlorine diffusion. The point of sodium bisulfite injection should be immediately upstream of the inlet isolation valve.
Activated carbon filtration may offer a more reliable means of breaking down chlorine. During manufacture, non-carbonaceous materials are burnt off, leaving porous granules with a substantial amount of pure carbon surface area. This has a high attraction for adsorbing almost any contaminant, including most organic materials and heavy metals, although there may be limited removal capacity for some contaminants that are shed into the effluent water.
The breakdown of chlorine by activated carbon involves an electrochemical reaction, which offers a high capacity for chlorine removal. The carbon gives up electrons to the chlorine atoms, forming innocuous chloride ions (Cl–) that remain in the water. In this reaction, oxygen atoms previously bonded with the chlorine atoms as hypochlorous acid (HOCl) now attach to the carbon surface. Because the carbon will also react with dissolved oxygen in the water, the carbon surface can become fully oxygenated. It will then lose its ability to remove additional chlorine, but this typically takes a few years with inlet chlorine concentrations less than 1 mg/L.
When ammonia is present naturally or when added by a municipality, chlorine will chemically bond with the ammonia to form monochloramine (NH2Cl) or possibly dichloramine (NHCl2). The chloramines are not as chemically reactive so require more carbon volume for their breakdown. A catalyzed carbon media is available at an increased cost that improves the carbon reactivity with chloramines and reduces the need for oversizing the carbon filters.
Carbon system valves cannot leak or otherwise bypass. They should be normally closed and driven by sufficient air pressure to not allow chlorinated water to reach the RO system.
Maintenance is critical to the success of either reducing-agent injection or carbon filtration. It is recommended that the activated carbon media be replaced annually or as based on an increase in its effluent concentration of TOC, to prevent the shedding of biological particles into the RO system.
Over-injection of sulfite will cause increased breakdown of dissolved oxygen in the water. This will increase the potential for heavy growth of slime-forming species of bacteria that can quickly foul an RO system if there is a sufficient concentration of organic food in the water source. This potential can be minimized by maintaining a residual sulfite concentration that is greater than zero but less than 2 mg/L as sodium sulfite, measured using a low-level test with sensitivity of 1 mg/L or less. As long as the sulfite concentration is greater than zero and it is well mixed into the feedwater, free chlorine will not be present.
 Scale formation
Scale formation
There is typically at least one salt in any natural water source that will concentrate beyond its solubility and potentially form scale (Fig 3). Preventing scale formation should not be a major challenge unless the water source has an unusually high concentration of a slightly soluble salt, or unless the RO is being operated with an unusually high-percent permeate recovery.
Scale formation may be prevented by injecting an acid into the inlet water, by softening the water, or by injecting a chemical scale inhibitor. Usually the least expensive method is a scale inhibitor, which slows the rate at which the salt crystals grow when their solubility is exceeded.
Acid injection prevents calcium carbonate scale formation, but leads to an extremely high concentration of carbon dioxide. Carbon dioxide is not removed by the RO system and will place a high removal demand on downstream ion exchange processes. Also, acid injection alone will not offer much protection against the formation of sulfate or certain other scales.
Softening offers several advantages, but suffers from high capital and operating costs, unless there are particularly low concentrations of calcium and magnesium hardness in the water. The softener also will remove other potential scale-forming ions, like strontium and barium, and will remove metals that would otherwise foul the RO system (for example, iron, manganese, and aluminum). But the softening resin will also foul with the metals and then require periodic chemical treatment.
Scale-inhibition chemical suppliers often will use software programs to estimate the potential for scale formation. These programs will predict the concentrations of salts that will be present in the RO concentrate stream, as well as its pH, to determine how much scale-inhibition chemical will be needed.
The potential for silica scale is common with certain ground-water sources in the West. Inhibition formulations have shown varied success. Maintaining warmer water temperature will improve silica solubility, as will changing the water pH. Increasing pH is a common strategy, although the water must first be softened to prevent hardness scale from forming when a caustic chemical is injected to raise the pH.
When using a scale inhibitor, it is critical to rinse the RO system of its increased concentration of dissolved salts whenever the RO shuts down. Otherwise, scale particles will grow and stick to the membrane surfaces during the shutdown. This rinsing process should be automated and is often performed with low-pressure inlet water. Low pressure reduces the RO permeation that tends to concentrate the dissolved salts.
A better rinse might be performed with pressurized permeate water if a line can be plumbed back to the RO from a permeate storage tank system. The permeate is biostatic. Its use will reduce the formation of biological solids within the RO while shut down.
 Membrane fouling
Membrane fouling
Fouling will not necessarily reduce RO membrane life if the RO is effectively cleaned. If the RO is allowed to foul too severely and cleaning is not effective, then the membrane will likely continue to lose performance.
It is common to include a filter housing on the RO inlet that contains 2.5-in.-diam cartridge filters whose pore size is nominally rated. The actual ability for removing smaller particles can vary greatly (Fig 4). Some (regardless of rating) will only protect the RO against large particles that might get caught within the membrane flow channels or damage the high-pressure pump. These are inexpensive and may last weeks before an elevated pressure drop indicates the need for replacement.
Tighter porosity filters that might remove more of the incoming suspended solids are more expensive and will also require more frequent replacement. Therefore, the use of these tighter filters becomes more economically viable if the concentration of suspended solids in the water has been minimized by upstream treatment.
Suspended solids often can be effectively reduced to reasonable concentrations for the downstream RO system with just a multimedia filter. Its inclusion in the RO water system might be sufficient to prevent a high RO fouling rate that could result in unmanageable cleaning requirements. Multimedia filters contain granules of two or more different types or sizes of sand, crushed rock, or anthracite (hard coal). Such a filter can be successful at removing most of the particles that make up the suspended solids if:
- It is sized for a downward flow velocity approaching 2 ft/sec.
- It has a lower collection lateral system designed to obtain uniform flow distribution across the media when the filter is operated at low flow velocity, while also allowing the entry of a sufficient backwash flow rate for a 40% bed expansion.
- The filter is backwashed before its previously removed smaller/fine particles are shed, which may occur before there is an appreciable buildup in filter pressure drop.
- After backwashing, the filter is forward-rinsed at its service flow rate until its effluent quality is acceptable (such as based on effluent iron concentration, turbidity, or SDI).
The preceding points do not provide all of the filter design requirements, but were chosen because these particular guidelines often are not followed (mostly because they would increase the filter’s cost).
Some water sources may contain unusually high concentrations of fine particles. In these cases, it may be necessary to send the water through large reaction tanks intended to give the particles more time to coagulate into larger particles that can then be more easily filtered.
An inorganic chemical coagulant (never a cationic polymer) may be added to the water upstream of the tank to speed the coagulation process. The coagulant will be most effective if it is first well-mixed with the suspended solids. If soluble metals (like iron or manganese) are present in the water source, some percentage will be oxidized by allowing the water to contact atmospheric air in the tank, although this percentage will be small.
A chemical oxidizer like chlorine (bleach) can be added to the water to oxidize the metals into their insoluble oxides (actually into their hydroxides when present in water) prior to coagulation.
Membrane filtration
Membrane filtration is becoming more common in various applications including pretreatment for RO systems. Membrane filtration often can provide water that is more consistently low in its concentration of suspended solids than that provided by a pressurized multimedia filter. Therefore, these systems may be used as an alternative to multimedia filtration, or possibly downstream to further polish the water and minimize RO fouling.
The most common configuration is hollow-fiber technology. Fibers of an inert polymer are extruded with a hollow internal region called the lumen. The fibers may be relatively fine/small in diameter where the inlet water passes through the outside of the fibers, and the fiber lumen. It then moves toward one end of the module for collection.
Because the fibers are tightly packed, flow movement around them is not uniform. Feedwater particles will come out of suspension on the membrane surface as the water goes through the fiber. They are not concentrated within a passing stream as the particles mostly would be with spiral-wound reverse osmosis, so there is no concentrate stream. The systems are simply operated at 100% recovery, except for the water losses from frequent backwashing with filtered water, resulting in an overall recovery of 90% to 95%.
There also are modules with larger fibers that use an inside-out service flow direction. The fatter fibers offer improved membrane surface flow characteristics for better distribution of the fouling solids, while the finer fibers offer the cost advantages of more membrane surface area in the modules.
The membrane filtration systems (Fig 5) should be sized to keep the fiber pressure differential (transmembrane pressure, TMP) relatively low to prevent compaction of solids against the fiber and into the fiber pore structure, and to reduce the potential for fiber breakage. This may mean sizing the fiber for a filtrate flux rate of 30 gal/ft²/day or less.

The fiber modules are backwashed using the filtrate water at a frequency of roughly once every 30 minutes, again to try to keep the solids from compacting and to prevent particles from getting forced into the pores and subsurface structure. Some manufacturers will reduce the backwash volume by knocking the solids free with compressed air.
Backwashing alone may not fully restore the original TMP. A chemically enhanced backwashing may then be required. If this fails to restore original performance, a circulated cleaning for an extended period of time may be needed.
Operation and monitoring
Large RO systems will include several membrane pressure vessels. These vessels will be staged so that the concentrated salt stream from one set of parallel-plumbed vessels will be plumbed into a smaller number of membrane vessels, then possibly plumbed to another stage with an even smaller number of vessels.
Staging is based on maintaining flow velocities sufficient to keep suspended particles moving and to assist dissolved salts in diffusing back into the bulk stream from the membrane surface (Fig 6).
RO systems usually are operated by adjusting the membrane feed pressure as needed to achieve the desired RO permeate flow rate. This may be done with a variable frequency drive (VFD) to control the high-pressure pump motor’s rotational speed, or by using a throttle valve located directly downstream of the pump. With VFD control, the adjustment may be automatic. The RO system will also have a concentrate stream throttle system to achieve the desired concentrate flow rate. This may be a fixed system that uses an orifice plate, or more commonly a manual or automatic valve.
Along with the permeate and concentrate flow meters, pressure sensors are installed in the system piping to monitor the pressure entering the membrane elements, the concentrate pressure exiting the membrane, and possibly the pressures within the plumbing manifolds that connect the membrane vessel stages (Fig 7). A permeate pressure sensor may be needed, especially if there is significant or variable permeate backpressure on the system.
The electrical conductance of the water streams is used to monitor how well the RO is removing dissolved salts. The RO permeate water conductivity is monitored along with the makeup water conductivity.
A percent salt rejection for the system is calculated by subtracting the permeate conductivity from the feed stream conductivity and then dividing this value by the feed conductivity. The salt rejection percentage is probably the most commonly monitored performance variable.
Additional instruments may be needed if there is variability in the feedwater characteristics, or if a chemical is being added (Fig 8). If the water acidity changes naturally or through chemical addition, then the water pH should be monitored continuously.
The pH can have a dramatic impact on the RO salt rejection. If chlorine is being removed upstream, an online chlorine monitor or possibly an oxidation-reduction potential (ORP) monitor may be used to warn against its presence.
Key variables
Monitoring the percent salt rejection is important, but is limited in its ability to show the state of the RO membrane and is generally not a good gauge of membrane fouling or scale formation.
The relative ability for water to permeate the RO membrane can be tracked using a variable called the normalized permeate flow rate, which is the RO permeate flow rate standardized for the effects of operating pressures, dissolved salt content, and water temperature. The feed-to-concentrate pressure drop tracks the resistance to water passage through the flow channels of the various membrane elements. This value may be calculated for the entire RO vessel array, or if inter-stage pressures are available, it can be calculated for the individual vessel stages.
If flow rates are not kept constant when operating the RO, it will be necessary to standardize the pressure drop for the effect of changing flow rates in calculating normalized pressure drop values. This then allows direct comparison of these values over time regardless of whether any flow rates have changed (Fig 9).
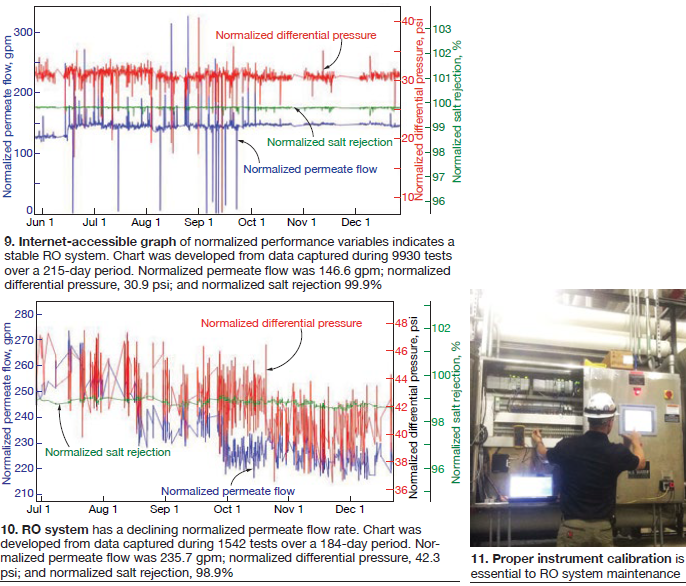
Small suspended particles or salt particles that coat the RO membrane surface will cause the RO normalized permeate flow rate to decline (Fig 10). Larger particles that get caught within the membrane flow channels and subsequently block the flow will cause the normalized pressure drop to increase.
If something in the water is chemically reacting with the RO membrane, the effect will likely be apparent in the normalized permeate flow rate, and possibly in the salt rejection. For example, if chlorine is allowed to come into contact with the RO membrane, the extent of membrane oxidation will be apparent as an increase in the normalized permeate flow rate soon followed by a decline in RO salt rejection.
A thorough understanding of the state of the RO system can thus be gained by routinely calculating and graphing the salt rejection and the two normalized performance variables. But their values may be misleading if any of the instrument readings are inaccurate. It is absolutely critical that monitoring instruments be routinely calibrated and repaired/replaced if in error (Fig 11).
When to clean
Chemical cleaning is a routine requirement for most RO systems. Frequency depends on the effectiveness of the pretreatment equipment.
As fouling solids or scale particles accumulate, their characteristics often change and they become more resistant to cleaning. Clay and biological materials will tend to compress against the membrane surface and become chemically resistant as water is squeezed out of their structure. Scale formations may change from being primarily calcium carbonate (relatively easy to clean) to calcium sulfate (difficult to clean).
The change in normalized RO performance variables can be used to determine cleaning needs. Most membrane manufacturers recommend cleaning before these variables change by about 15%.
Certain types of fouling solids or scaling salts may have a substantial impact on permeate quality. Aluminum salts may come out of suspension as a fouling particle, only to re-dissolve if the water acidity changes. This may then result in increased aluminum passage from the membrane surface through the membrane and into the permeate/purified water.
Calcium carbonate scale may leach a relatively high concentration of calcium carbonate through the membrane into the permeate stream and affect the conductivity. Most other fouling solids will not have a significant impact on RO salt rejection unless the fouling is extreme.
How to clean
Membrane cleaning involves passing a cleaning solution through the membrane system at conditions that promote the dissolution or delamination of the fouling solids from the membrane surface or from the spacing material along the membrane flow channels. The optimum solution will depend on the particular fouling solids or scale particles, and the relative ability to clean will often be limited by membrane chemical tolerance.
Most strong oxidizing agents that would typically be effective in cleaning biological solids are not going to be compatible with the RO membrane. There will also be limits to the pH extremes that should be used. In addition, while higher temperature will increase the rate of cleaning, the solution temperature will be limited to below 105F or as designated by the membrane manufacturer.
The most critical characteristic of a cleaning solution is its pH. Acidic solutions are more effective in dissolving metals and scale formations, while alkaline (high pH) solutions are more effective in removing clay, silt, biological, and other organic solids. Strongly acidic solutions may stabilize biological solids and therefore should not be used as a first cleaning step. Finishing a cleaning with a strongly acidic solution will tend to leave the membrane with increased rejection characteristics but somewhat reduced permeate flow, while finishing with a strongly alkaline solution will have the opposite effect.
The addition of specific cleaning agents often improves the solution’s cleaning abilities. A chelating agent assists in pulling out metals from the fouling solids, while surfactants/detergents improve the solution’s ability to penetrate the fouling solids and suspend oily substances. The use of surfactants may reduce cleaning time but will increase the time required for rinse up.
When the fouling solids are causing a flow restriction increasing normalized pressure drop, high cleaning flow rates (within the membrane manufacturer’s guidelines) through the membrane feed channels will cause agitation that will assist in breaking up the deposits. When the solids coat the membrane surface and reduce the normalized permeate flow rate, the delamination of these solids will be most easily achieved if water is not permeating through the membrane during the cleaning process and creating a force that holds the solids to the surface. This means cleaning at low pressure.
Achieving a high cleaning flow rate that is balanced throughout all of the membrane vessels usually requires that each vessel stage be cleaned separately. This also helps minimize the pressure required to push the solution through the elements. Cleaning solution is therefore pumped at high flow rates, as recommended by the membrane manufacturer. It is pumped at the maximum pressure required to achieve the target flow rate, but may be limited to 60 psi to reduce the potential for crushing or otherwise damaging the membrane elements.
The solution is directed in the normal feed-end direction of flow and the exiting concentrate stream is then returned to the cleaning tank at minimal backpressure. The flow direction may occasionally be reversed so that the solution enters the concentrate end of the stage when fouling solids are blinding the face of the lead-end membrane elements.
There may be a small flow of permeate that should also be returned to the cleaning tank using a separate line. In spite of its low apparent flow rate, the permeate should never be valved off because this may put certain membrane elements at risk of physical damage.
Data should be recorded during the cleaning process. With membrane surface fouling, it is difficult to gauge when original performance has been restored until the unit is rinsed and operated normally. If the fouling solids were causing an increased pressure drop in the RO, then the cleaning inlet pressure can be used as a measure of cleaning progress. If the pressure keeps declining, the cleaning is still removing fouling solids. If the fouling is severe, it may require a number of hours of circulation before the inlet pressure stabilizes.
Cleaning success is confirmed when the normalized pressure drop and normalized permeate flow rate return to their startup values. CCJ